Recruitment and screening
The study’s inclusion criteria were being born in 1975 or earlier and residing in Iceland on the 9th of September 2016, as registered in the Icelandic National Registry. Eligible individuals were invited to participate in the iStopMM study (n = 148,711). A letter containing a detailed information brochure and consent form was mailed to them and an extensive campaign on social and conventional media was launched introducing the study to the Icelandic public. This campaign was followed by phone calls to those who had not yet signed up for the study.
Participants could provide informed consent through three different mechanisms:
- returning a signed informed consent form by mail,
- registering electronically using a participation code included in the invitation letter, or
- through a secure internet gateway provided by the Icelandic government (island.is), which is accessible to all residents through a secure electronic authentication process. The only exclusion criterion was previously known LP, other than MGUS.
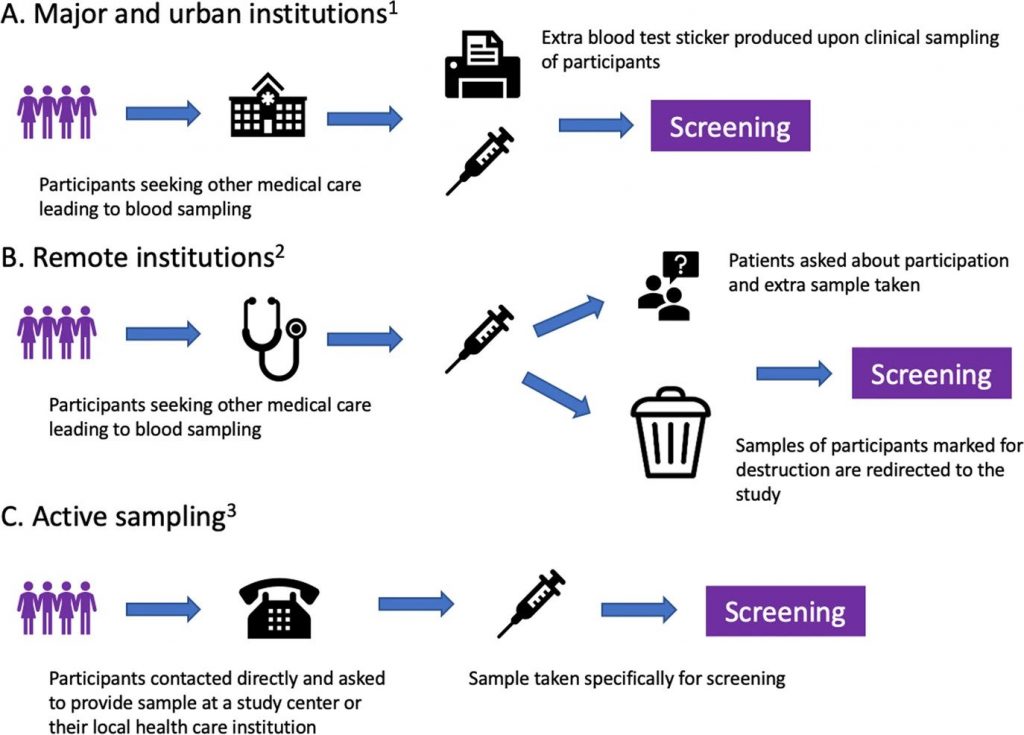
After enrollment, serum samples for screening are collected alongside the collection of blood during clinical care in the universal Icelandic healthcare system, including blood banks (Fig. 1). The study team in collaboration with Landspítali—The National University Hospital of Iceland (LUH), developed an electronic system linking participant data to the central laboratory network of all major and smaller urban healthcare institutions, which covers at least 92% of all Icelandic residents. The system notifies healthcare workers to take an extra blood sample for the study at the point of clinical blood sampling.
For smaller rural institutions and private clinics, a manual system was developed whereby laboratory technicians crosslink left-over samples marked for destruction to registered participants and in some cases ask their patients if they are participants in the study and draw an additional sample for the study. To capture samples from participants who do not require clinical blood sampling, an active sampling drive was initiated after three years of passive sample collection.
Fig. 1: Methods of blood sample acquisition.
A and B describe passive sampling starting during the fall of 2016, and C describes active sampling beginning during the fall of 2019. 1: Reykjavik Capital Area, Akureyri, Ísafjörður, Reykjanes Peninsula, Akranes, Healthcare Institution of Northern Iceland, Healthcare Institution of South Iceland, blood banks 2: Neskaupsstaður, Healthcare institution of West Iceland, Healthcare Institution of East Iceland. 3: Available for all Icelandic residents.
All samples are sent to the clinical laboratory at LUH in Reykjavik, Iceland where serum is aliquoted into identical sample tubes and assigned an anonymous study identification number. The laboratory uses TC automation and aliquoter (Thermo Scientific®, MA, USA) for sample handling. Samples are then sent to The Binding Site laboratory in Birmingham, UK where all samples are screened for M protein by capillary zone electrophoresis (CZE; Helena Laboratories, Texas, USA) and for FLC, immunoglobulins (IgG, IgA, and IgM), and total protein by Freelite® and Hevylite® assays performed on an Optilite® turbidimeter (The Binding Site Group Ltd, Birmingham, UK). Immunofixation electrophoresis (IFE; Helena Laboratories, TX, USA) is performed on samples with clear or suspected M protein bands by CZE and/or abnormal FLC results. The CZE and IFE gels are assessed independently by at least two experienced observers.
Randomization and study arms
Participants with an M protein or pathological FLC results are considered eligible for the RCT and are randomized into three study arms in a dynamic, non-predetermined manner (Fig. 2). To avoid skewed distribution of high-risk MGUS and LC MGUS, randomization is carried out by blocks of having an M protein >1.5 g/dL and having LC-MGUS. Participants in arm 1 are not informed of their MGUS status and continue to receive conventional healthcare as if they had never been screened. Arm 2 follows current guidelines for follow-up, stratified by low and non-low risk MGUS1. Arm 3 follows a more intensive strategy that is not risk-stratified (see below).
Fig. 2: A flowchart outlining the study design for screening and randomization of individuals with MGUS.
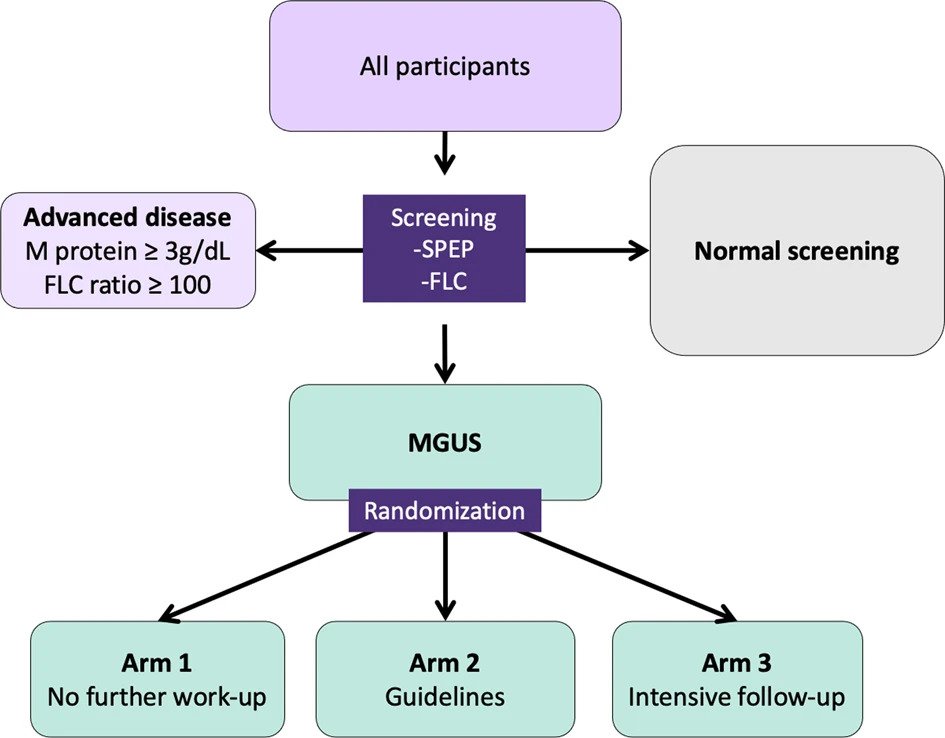
Participants with an M protein ≥3.0 g/dL or an FLC ratio ≥100 are not eligible for randomization but are all called in for evaluation since they have, by definition, more advanced disease than MGUS1,8,10. Participants with previously diagnosed MGUS cannot be randomized to arm 1, as they are aware of their MGUS status, and are thus randomized to arms 2 or 3 and will not be included in comparisons with arm 1.
Initial assessment and follow-up
Initial assessment and follow-up of participants in arms 2 and 3 and participants diagnosed with more advanced disease (SMM, SWM, MM, AL, or other LP) at screening is performed in the iStopMM study clinic in Reykjavík, Iceland. Temporary clinics are also regularly established in Akureyri, Ísafjörður, Húsavík, and Egilsstaðir for complete geographical coverage. All participants who are called into the clinic are seen by specialized study nurses and those with more advanced disease are also seen by a physician. The participants undergo a clinical interview and thorough clinical examination and are given detailed oral and written information about their diagnosis and prognosis.
Participants in arm 2 with non-IgM MGUS or LC-MGUS are stratified by having low-risk MGUS or not. These participants are then followed according to guidelines including plain skeletal surveys and bone marrow sampling for those with non-low risk MGUS or when clinically indicated1. All participants in arm 3 follow an intensive follow-up schedule regardless of risk, including bone marrow sampling and whole-body low-dose computerized tomography (WB-LDCT). Participants in arm 2 and 3 with IgM MGUS undergo a computerized tomography (CT) of the abdomen. Diagnostics and follow-up intervals for arms 2 and 3 are shown in Table 1. Participants with smoldering or active disease at baseline or later are followed according to guidelines. This includes intensive follow-up every 4 months or sooner if clinically indicated with annual bone marrow samples and WB-LDCT, as well as magnetic resonance imaging (MRI) if no bone lesions are seen on WB-LDCT. Participants who develop intermediate to high-risk SMM, MM, or other related disorders that require treatment are offered participation in a treatment trial (ClinicalTrials.gov identifier: NCT03815279) or referred to the hematology unit at LUH or Akureyri Hospital for evaluation, treatment, and follow-up.
To detect AL, urine samples are tested for proteinuria in participants visiting the study clinic. In addition, participants in arm 3 and those with more advanced disease are tested for cardiac markers (Table 1). Those with significant proteinuria and decreased kidney function of unclear etiology are referred to a nephrologist for further evaluation. Those with abnormal cardiac markers not explained by known comorbidities are referred to a cardiologist for clinical evaluation and echocardiography. Bone marrow biopsies are stained with Congo red for the presence of amyloid fibrils in all these cases and another testing for AL is performed as clinically indicated.
After each visit, participant’s test results and clinical findings are thoroughly reviewed by the primary investigator and the clinic staff with respect to their disease status and progression at regular clinical decision meetings. Additional testing including repeat bone marrow sampling, imaging, blood sampling, or clinical evaluation is ordered as clinically indicated at or between protocol visits. Diagnoses of SMM, MM, SWM, WM, AL, and other LP are made according to current diagnostic criteria1,8,26,27.
Imaging
Plain radiographs, WB-LDCT, and CT of the abdomen are performed in LUH and Akureyri Hospital. MRI is performed in LUH and Akureyri Hospital. All radiological images are reviewed independently by two physicians, one in specialty training and a senior radiologist at LUH. The radiological assessments are blinded and any discordance in findings is discussed and solved by the two physicians.
Bone marrow samples
Bone marrow sampling is performed by study nurses that have been trained, both locally and in an accredited facility in the United Kingdom (The Royal Marsden Hospital, London, UK). Samples are collected as bone marrow smears and as trephine biopsies. Bone marrow smears are stained with Giemsa stain and jointly evaluated by two senior hematologists at LUH reporting the percentage of BMPCs or lymphoplasmacytic lymphocytes, lymphoid infiltrates, and sample quality. Trephine biopsies are stained with hematoxylin and eosin, as well as for CD138 before being evaluated by two senior hematopathologists at LUH. The sample with the higher percentage of BMPCs/lymphocytic infiltration at each sampling time is used to guide follow-up.
Questionnaires
Immediately following informed consent, participants were asked to complete questionnaires on psychiatric symptoms (e.g., anxiety and depressive symptoms) and life satisfaction to establish a baseline prior to screening28,29,30. Throughout the study period, all participants, regardless of screening status, are asked to complete the same questionnaires electronically at predefined intervals, as well as additional questionnaires on psychiatric health, pain, neuropathic symptoms, and more (Table 2).
Table 2 Questionnaires sent to participants by email or answered at the study clinic.
Those who visit the study clinic (arms 2 and 3, and individuals with more advanced disease) answer more extensive questionnaires at each clinic visit and annually. Those who are randomized to arm 1 or are screened negative continue to receive the same annual questionnaires. One-time questionnaires, e.g., baseline characteristics, employment history, resilience, social support, and adverse childhood experiences are sent to all participants by email (Table 2).
Currently, 72 918 (90%) of all participants have provided their email addresses. All non-valid email addresses are reviewed by study staff and participants who visit the study clinic are asked to provide a valid email. Participants are reminded to answer the questionnaires in three separate emails.
Registry crosslinking
Several national healthcare-related registries exist in Iceland that can be accurately crosslinked using a government-issued national identification number. Data from these registries are linked to all participants in the iStopMM study at least twice each year.
The following registries are linked to the study datasets:
(1) The Icelandic Cancer Registry includes information on all cancers diagnosed in Iceland. It has been mandatory for all physicians and pathologists to register diagnoses of cancer since 1955 and it is virtually complete with high diagnostic accuracy and timeliness31;
(2) The Icelandic Causes of Death Registry includes all deaths in Iceland including the date and the presumed causes of death. Registration has been mandatory since 1971;
(3) The Icelandic Prescription Medicines Registry includes all prescriptions, including whether the prescriptions were filled or not. in Iceland since 2002;
(4) The Icelandic Hospital Discharge Registry includes all inpatient admissions in Iceland from 1999 with the dates of admission and discharge, as well as international classification of diseases (ICD) codes for the diagnoses made by treating physicians. The registry also includes outpatient visits at hospitals, including emergency rooms since 2010;
(5) The Icelandic Registry of Primary Health Care Contacts includes all primary care visits and registered ICD-coded diagnoses for all primary care encounters in Iceland since 2004;
(6) The Icelandic Central Laboratory Database comprises laboratory test results from all major clinical laboratories in Iceland stored in a central database since 1999, including all blood tests for participants prior to participation and during follow-up in the study;
(7) All medical records at LUH, the only tertiary care medical center in Iceland and the general acute care hospital for the vast majority of Icelandic residents. This includes clinical notes, anthropometric data, written radiology and pathology reports, microbiology and virology test results, and all other documented clinical data.
Biobanking
Blood samples drawn at each clinic visit are biobanked including cell-free plasma, serum, and plasma. Bone marrow samples are collected for biobanking in parallel to bone marrow sampling. Urine and blood in Blood-RNA tubes (PAXgeneTM) tubes and in mononuclear cell preparation tubes (BD Vacutainer® CPTTM) are collected at sparser timepoints (Table 3). Samples are processed on-site and aliquoted at the study laboratory in Reykjavík, Iceland, and bone marrow samples separated into plasma and buffy coats. The bone marrow buffy coats from non-IgM MGUS and LC-MGUS are further separated into a plasma cell-enriched CD138+ fraction and a CD 138− fraction by Magnetic-activated cell sorting (MACS) using CD138 MicroBeads and an autoMACS pro cell separator (Miltenyi Biotec, Bergisch Gladbach, Germany). All cell fractions are cryopreserved and stored in liquid nitrogen. Other biobanking samples are frozen and stored in a secure state-of-the-art robotic biobanking facility in Reykjavík, Iceland, and cataloged using unique study identification numbers.
Table 3 Biosamples included in the study biobank and when they are obtained from participants.
Study monitoring
A study monitor was appointed to review the study protocol and regularly assessed the conduction of the study for compliance with relevant good clinical practice (GCP) principles. An independent data monitoring committee was established including two clinicians and a statistician that are not associated with the study. Interim analyses assessing safety and efficacy data are performed biannually. Additional interim analyses are scheduled when 500 subjects with MGUS have been followed for 6 months and when 100 participants with MGUS have died. When participants who have been randomized have been followed for five years, or if interim analysis shows a difference in the overall survival between arm 1 compared to arms 2 and 3, arm 1 will be discontinued. At that time the participants in arm 1 are unblinded to their MGUS status and offered a choice between randomization to arms 2 or 3, or clinical follow-up in the Icelandic healthcare system.
Study endpoints
The primary endpoint of the study is the overall survival of individuals with MGUS receiving follow-up (arms 2 and 3) compared to those not receiving any follow-up within the study (arm 1) after 5 years of follow-up. Secondary endpoints are cause-specific survival due to MM or other LPs, psychiatric health and well-being, and cost-effectiveness of screening. In addition, study data will be crosslinked to registries and samples in the biobank providing a large dataset for future studies.
Assuming that 3360 individuals with MGUS are identified and the hazard ratio (HR) for the primary outcome is 0.81 as previously described32 the study has 77.2% power to reject the null hypothesis of HR = 1 at 5 years of follow-up and 89.3% power at 7 years of follow-up at an alpha level of 0.05.
